GDPH COVID-19 Provider Update
Starting Monday, January 11, 2021, adults aged 65+ and their caregivers will be eligible for COVID-19 vaccination as a part of phase 1A+. Continue to visit the DPH website to review the Georgia COVID-19 Vaccine Status Dashboard and obtain important vaccine-related information.
Current Vaccine Phase:
Effective Monday, January 11, 2021, the expanded list of individuals eligible for vaccination in Phase 1-A will include:
- Healthcare workers in clinical settings (e.g., nurses, physicians, EMS, laboratory technicians, environmental services)
- Staff and residents of long-term care facilities
- All law enforcement and fire personnel (including volunteer departments), and first responders
- Adults aged 65 and older (and their caregivers as applicable)
Helpful Reminders:
- Providers are not required to hold supply in reserve for 2nd doses. For example, if you received 100 doses and administered 50 doses, continue to administer the remaining 50 doses to new patients who meet the above Phase 1A+ criteria. Providers must place orders for 2nd doses. Refer to the attached Vaccine Ordering Guidance for more information.
- Use this link to complete the survey to let us know if you are willing to be a mass vaccination site. Responses must be received by January 13, 2021 at 5:00pm.
- Providers must establish and enforce accountability measures related to vaccine distribution. Your orders are dependent on available supply allocation for Georgia and your timely and accurate reporting of inventory and doses administered to GRITS. As required by Georgia law and your vaccine provider agreement these immunizations must be reported within 24 hours of administration. Late or forgone reporting puts provider supply at risk and Georgia residents at risk of transmission and exposure to COVID-19, and additional loss of life. If you are not using GRITS or are having difficulty navigating the system, staff are available to assist Monday – Friday, 8 a.m. to 5 p.m. by calling (866) 483 -2958 or emailing dph-gaimmreg@dph.ga.gov.
- Vaccine storage unit temperatures must be monitored regularly and recorded at the start of each workday. Always record minimum/maximum temperature, date, time, name of person checking/recording temperature, actions taken if a temperature excursion occurred. *Temperature records must be kept for a minimum of three years, or longer if required by your jurisdiction.
General Information:
- Interim Considerations: Preparing for the Potential Management of Anaphylaxis at COVID-19 Vaccination Sites – Locations administering COVID-19 vaccines should adhere to CDC guidance for use of COVID-19 vaccines, including screening recipients for contraindications and precautions, having the necessary supplies available to manage anaphylaxis, implementing the recommended post vaccination observation periods, and immediately treating suspected cases of anaphylaxis with intramuscular injection of epinephrine.
- Providers should not contact Pfizer or Moderna to cancel a vaccine request. If a provider is not able to store a vaccine delivery, they should immediately notify the Georgia Immunization Program by calling 404 657-3158 and also notify their local health department. Public health staff will work to locate a facility where the vaccine can be transferred. The facility that initially requested the vaccine should accept the shipment. Do not open the shipping container. The vaccine should be kept in a secure location until it can be picked up and moved to another location
- Vaccine providers with available vaccine should vaccinate members of the community meeting any of the above Phase 1A+ criteria, not just those within their own staff or facility. Due to limited vaccine supply availability, unless they are in one of the above populations, spouses and family members are not eligible for vaccine administration at this time. The Georgia Department of Public Health will notify COVID vaccine providers when to move to the next phase. Moving to additional phases without approval from DPH is a violation of the vaccine provider agreement.
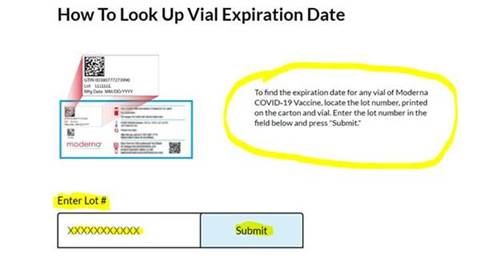
- Visit the Moderna website and scroll to the How To Look Up Vial Expiration Date section to locate vaccine vial expiration date.